

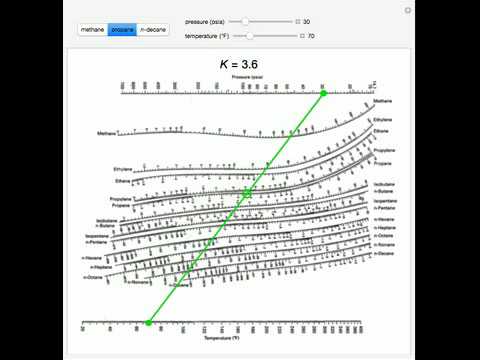
When a binary mixture is distilled, complete separation of the two components is rarely achieved. As the value of α increases above 1, separation by distillation becomes progressively easier.Ī liquid mixture containing two components is called a binary mixture. When the volatilities of both key components are equal, α = 1 and separation of the two by distillation would be impossible under the given conditions. That means that α ≥ 1 since the larger K value of the more volatile component is in the numerator and the smaller K of the less volatile component is in the denominator. Thus, a K value (= y / x) for a more volatile component is larger than a K value for a less volatile component. When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. = K commonly called the K value or vapor-liquid distribution ratio of a component = the vapor-liquid equilibrium concentration of component j in the liquid phase = the vapor-liquid equilibrium concentration of component j in the vapor phase = the vapor-liquid equilibrium concentration of component i in the liquid phase
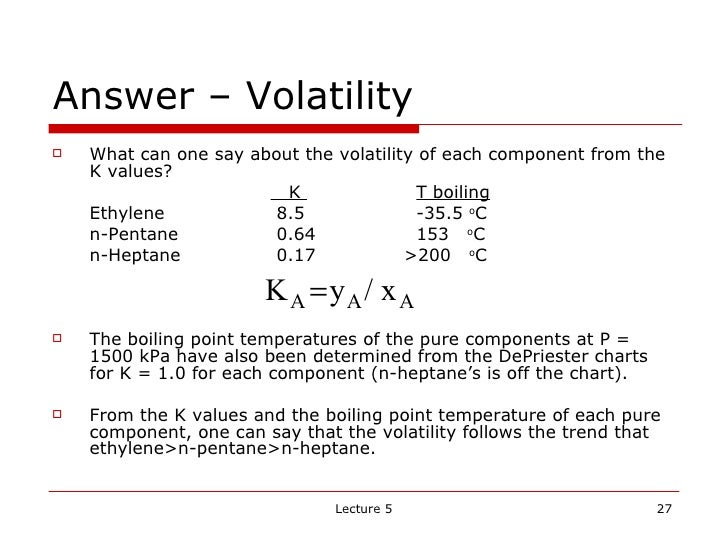
= the vapor-liquid equilibrium concentration of component i in the vapor phase = the relative volatility of the more volatile component i to the less volatile component j Relative volatilities are not used in separation or absorption processes that involve components reacting with each other (for example, the absorption of gaseous carbon dioxide in aqueous solutions of sodium hydroxide).įor a liquid mixture of two components (called a binary mixture) at a given temperature and pressure, the relative volatility is defined as
#HOW TO READ A DEPRIESTER CHART SERIES#
Relative volatilities are used in the design of all types of distillation processes as well as other separation or absorption processes that involve the contacting of vapor and liquid phases in a series of equilibrium stages.


 0 kommentar(er)
0 kommentar(er)
